Hello Engineers, In today’s article, I am going to explain in a bit brief about Lithium-ion vs. Lead-Acid batteries and also their merits &demerits. How are they different? How is the one better than another. Their chemical and electrical properties. Also Lithium-Ion vs. Lead-acid batteries. so let’s get started. Lithium-ion vs. lead-acid Tabular difference at the end.
Lithium-ion vs. lead-acid batteries.
Lithium-ion Battery
A lithium-ion battery or Li-ion battery is a type of high-quality rechargeable battery commonly used for rechargeable portable electronics devices like Walkie-Talkies, Hands-free landlines or toys, drones, etc and also used in Electric vehicles, Laptop battery packs, rechargeable power tools like mini power drills, etc.
In these batteries ions of lithium travel from negative electrodes to positive electrodes inside the battery through the electrolyte and during discharge or load connected and vice versa(Positive to the negative electrode ) during charging. Lithium-ion contains calculated lithium compounds at the positive side of the electrode and Graphite to the negative electrode.
These are very high-quality batteries that have high power density and a very low self-discharge which is very good quality. Below is a diagrammatical explanation of the basic structure of lithium-ion batteries.
Lithium-ion battery diagram

However, these batteries can be dangerous due to the flammable property of its electrolyte or if it’s recharged incorrectly or damaged, then it can explode. Usually smartphones these days use lipo batteries.
Li-po batteries, as well as lithium-ion, are needed to be recharged very safely and with an appropriately designed charger circuit that has an only adequate amount of voltage and current outputs to recharge these batteries. These batteries come in a variety of models.
The energy density of lithium-ion is very high as compared to that of the standard nickel-cadmium or Ni-Mh There is potential for higher energy densities. The load characteristics are reasonably good and behave similarly to nickel-cadmium in terms of discharge. The high cell voltage of 3.6 volts allows battery pack designs with only one cell.
Most of today’s mobile phones run on a single cell. But for a nickel-battery pack it will be required three 1.3 volt cells connected in series and that too, will not provide efficiency like lithium-ion.

Lithium-ion battery Charging
The charging instructions and basic requirements are the same for lithium-ion and lithium-polymer.Charging lithium ion phosphate 3.2 volt battery/cells is identical, but the constant voltage supply is upto to 3.65 volts. Charging a lithium-ion with safety is very necessary and complex. The basic algorithm is to charge at constant current supply until the battery reaches 4.2 V per cell and hold the voltage at 4.2 volts until the charge current has dropped to 10% of the initial charge rate. The termination condition is the drop in charge current to 10%. The top charging voltage and the termination current varies slightly with the manufacturer. This requires a powerful and automatic cutoff charger circuit to charge the battery effectively with safety.
It is important to note that trickle charging can’t be performed over lithium batteries. The Li-ion chemistry cannot accept an overcharge without causing damage to the cell, possibly plating out lithium metal and becoming hazardous. Every lithium-ion battery pack should be charged by keeping all the cells balanced and preventing them from being over-discharged. This is usually done with a safety board that monitors the charge and discharge of the batteries and prevents the battery from getting damaged or getting exploded.
Lithium-ion battery advantages
- Relatively low self-discharge – self-discharge is less than half that of nickel-based batteries.
- Low maintenance required.
- Very high current output.
- Reliable
- High efficiency
- Compatible Size
Lithium-ion battery limitations
- Very much expensive
- High manufacture cost
- Sophisticated usage topologies
- Improper connection or Improper charging leads to damage easily or can cause human injury too.
Lead Acid Battery
A lead-acid battery is the most widely used and another powerful rechargeable battery with high current output and good efficiency as compared to Ni-Cd or Ni-Mh.
Let’s first understand a bit about Lead-acid Battery. The lead-Acid battery is a kind of Storage battery/ Rechargeable battery. And as we know, the battery is a group of cells, so the same is with it. Each cell in a Lead -Acid battery is having a Spongy / Porous Lead(Pb) electrode acting as a negative terminal and the Positive electrode made up of Lead Peroxide(PbO2) dipped in dilute Sulphuric Acid(H2SO4) Electrolyte in a container.
The battery comes in two types:-
- Normal Lead Acid/Automobile grade
- Sealed Lead Acid battery /maintenance-free.
A battery is a group of cells and each lead-acid cell is consisting of: Plates, Separator and Electrolyte with Hard Plastic with a hard rubber case
Lead acid battery chemistry
- PLATE: The plate of the lead-acid consists of some form of a grid that is made up of lead and the active material.
- SEPARATOR: Separators between the positive and negative plates prevent short-circuit through physical contact, mostly through dendrites (“treeing”), but also through the shedding of the active material. Separators allow the flow of ions between the plates of an electrochemical cell to form a closed circuit. Wood, rubber, glass fiber plastic are materials that can be used to make separators.
- ELECTROLYTE: The sulfuric Acid( The diluted sulfuric acid H2SO4 molecules break into two parts when the acid dissolves).
The Active Components of a Lead Acid Battery
- Lead peroxide (PbO2) – It forms the positive active material. The PbO2 are dark chocolate broom in color.
- Sponge lead – It forms the negative active material. It is grey in color.
- Dilute Sulfuric Acid (H2SO4) Dilute – It is used as an electrolyte. It contains 31% of sulfuric acid.
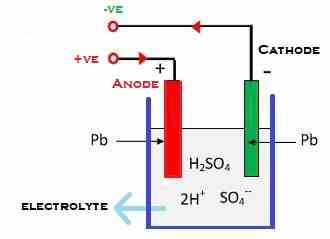
Lead-acid battery Working/ charging
- The diluted sulfuric acid H2SO4 molecules break into two parts when the acid dissolves. It will create positive ions 2H+ and negative ions SO4-.
- As we told before, two electrodes are connected as plates, Anode, and Cathode. The anode catches the negative ions and the cathode attracts the positive ions.
- This bonding in Anode and SO4– and Cathode with 2H+ interchange electrons and which is further reacted with the H2O or with the water (Diluted sulfuric acid, Sulfuric Acid + Water).
- For charging a lead-acid battery we need a charging voltage bit higher than the nominal battery voltage.
- for example, to charge a battery of 12v (any Ah), we need to apply at least 13.5v DC to the battery terminals for charging.
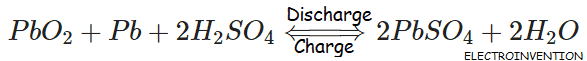
- During the Discharge of a battery, there is the formation of lead sulfate crystals at both the negative and positive terminals, as well as the release of electrons due to the change in valence charge of the lead. This formation of lead sulfate uses sulfate from the sulfuric acid electrolyte surrounding the battery. Batteries create sulfation each time they are used (discharged – recharged). If they are overcharged or undercharged or are discharged for a long time, they will rapidly develop sulfate. Even if a battery is stored fully charged, sulfate will form unless a desulfation battery charger is used.
- The charging reaction converts the lead sulfate at the negative electrode to lead. At the positive terminal, the reaction converts the lead to lead oxide. As a by-product of this reaction, hydrogen is evolved. During the first part of the charging cycle, the conversion of lead sulfate to lead and lead oxide is the dominant reaction. As the charging goes on and most of the lead sulfate is converted to either lead or lead dioxide, the charging current electrolyzes the water from the electrolyte and both hydrogen and oxygen gas are evolved, a process known as the “gassing” of the battery.
Desulfation is a reversal process to remove sulfation from a dead or sulfated battery. Desulfation is to restore a battery by breaking and removing the lead sulfates crystals from the battery electrodes, either fully or partially. Desulfation either restores the battery to its full potential or up to 60-70% sometimes. Read More on the topic click here
Advantages Of Lead Acid batteries
- Cheap /cost-effective.
- Easily available and feasible.
- Easy to use and Charge.
- Lesser efforts required to design a charger for them.
- Good for Medium and Large amount of energy storage.
- Its electrolyte is not flammable.
- The batter is designed well.
Disadvantages Of Lead Acid batteries
- Low energy Density
- Short Life cycle
- Causes Lead pollution
- Short Life Cycle, as compared to lithium-ion battery
- need to be regularly charged
Tabular Difference Between Lithium-ion and lead acid battery
| Lead-acid | Lithium-ion |
| 1. Self-discharge is Low as compared to Ni-Cd but higher than Lithium-ion. | 1. Relatively low self-discharge as compared to others. |
| 2. Regular charges and filling distilled water required regularly. | 2. Low maintenance required. |
| 3.Higher but low as compared to lithium-ion. | 3. Very high current output. |
| 4. Efficiency is less than lithium-ion. | 4. High efficiency |
| 5. Size is a problem | 5. Compact Size |
| 6. Cost is Lower as compared to Lithium-ion. | 6.Higher cost. |
| 7. The charging process is not that much sophisticated. | 7.Sophisticated charging. |
I hope Your people liked this post. Stay tuned for other interesting posts. Also, read other articles for making electronics projects and gadgets. Also, read this article on:-
Automatic Battery charger circuit with auto cutoff
recovering a dead lead acid battery and desulfator circuit.


3 Replies to “Lithium-ion vs. Lead-Acid batteries – Detailed Comparison”